Barbourofelis loveorum

Quick Facts
Common Name: Loves’ false sabercat
Barbourofelis loveorum is an extinct sabertoothed carnivore that weighed approximately 150 lbs, or about the size of a modern jaguar.
It is believed that the Barbourofelis loveorum preferred a thickly wooded habitat, and was an ambush predator that may have been able to bring down prey much larger than itself.
Fossils of Barbourofelis loveorum have been found in Florida, California, and Oklahoma. A much larger, tiger-sized species, Barbourofelis fricki, is known from Nebraska and Nevada.
Age Range
- Late Miocene Epoch; latest Clarendonian [Cl3] to early Hemphillian [Hh1] land mammal ages
- About 8 to 9.5 million years ago
Scientific Name and Classification
Barbourofelis loveorum Baskin, 1981
Source of Species Name: The species was named after Ron and Pat Love, owners of the Love Bone Bed (Baskin, 1981).
Classification: Mammalia, Eutheria, Laurasiatheria, Carnivora, Feliformia, Aeluroidea, Barbourofelidae
Alternate Species Names: Barbourofelis lovei
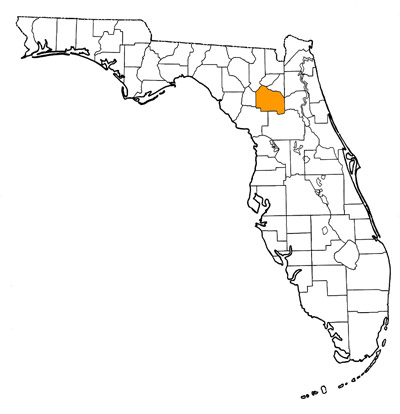
Overall Geographic Range
While the type locality is the very late Clarendonian Love Bone Bed of Alachua County, Florida, other specimens referred to this species came from the late Clarendonian of California and the early Hemphillian of Oklahoma (Baskin, 1981), so its distribution extended across the entire continent of North America.
Florida Fossil Occurrences
Florida fossil sites with Barbourofelis loveorum:
- Alachua County—Love Bone Bed
Discussion
Barbourofelis loveorum is an extinct sabertoothed carnivore. The genus Barbourofelis is traditionally attributed to the family Nimravidae, an extinct group of carnivorous mammals with many characters in common with the true cats, the Felidae. Early evolutionary analyses placed Barbourofelis (and closely related taxa such as Albanosmilus, Prosansanosmilus, and Sansanosmilus) into a subfamily of Nimravidae, the Barbourofelinae (e.g, Bryant, 1991). However, the results of more recent studies place these genera in their own family, separate from nimravids, the Barbourofelidae (Morlo et al., 2004; Robles et al., 2013). In the historical biogeographic scenario proposed by Robles et al. (2013), Albanosmilus dispersed from Asia into North America and is represented by the middle Miocene species Albanosmilus whitfordi. Later in the middle Miocene, this species or a similar one gave rise to the genus Barbourofelis, and of the four recognized species, Barbourofelis loveorum is the most primitive member of the genus. Barbourofelis may have dispersed back into Asia in the late Miocene, based on the presence of a highly evolved form known from Turkey (Robles et al., 2013).

Baskin (1981) originally named the species Barbourofelis lovei. The species name was amended by Hulbert (1992) to Barbourofelis loveorum because it was stated in the original paper that the species was named after Ron and Pat Love. According to Article 31.1.2 of the code of the International Commission on Zoological Nomenclature, the suffix –i is added to form a species name only if it is based on a single man. The suffix –orum is the correct ending when a species is named after two or more men, or after any combination of men and women (which is the case here).
Barbourofelis loveorum is smaller in size than Barbourofelis fricki from the early Hemphillian of Nebraska, with a less pronounced mandibular flange and larger upper third premolar (P3). However, Barbourofelis loveorum is larger in size than Barbourofelis morissi from the late Clarendonian of Nebraska, with a smaller P3 and more “excavated” mastoid process (Baskin, 1981). Meachen-Samuels (2012) estimated the average weight of Barbourofelis loveorum to be approximately 70 kg (150 lbs), or about the size of a modern jaguar.
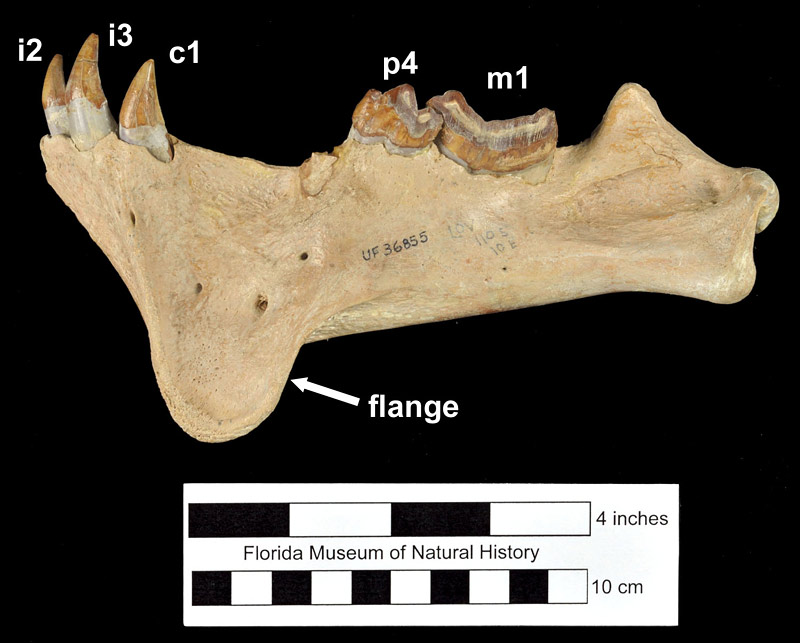
In terms of ecomorphology, Barbourofelis loveorum (along with all other members of its genus) is described as a “dirk-toothed” sabertooth. “Dirk-toothed” sabertooths are characterized by having longer, thinner upper canines relative to “scimitar-toothed” sabertooths which have wider, shorter upper canines that can be coarsely serrated (Martin, 1980; Meachen-Samuels, 2012). Examples of both types of sabertooths are known in the families Nimravidae and Felidae, while all members of the Barbourofelidae have the dirk-toothed morphology. Along with these differences in the upper canine, dirk-toothed forms generally have stouter, more powerful limbs, especially the forelimb, while scimitar-toothed forms have longer, more slender limbs. Dirk-toothed forms such as Hoplophoneus (a nimravid), Smilodon (a felid), and Barbourofelis (a barbourofelid) are thought to have been predators that waited in ambush to procure prey (Martin, 1980; Therrein, 2005). The envisioned scenario is that their heavier, sturdier build (that presumably would not have been conducive to a fast running prey-capture style due to their weight) was used to ambush and reduce the struggle of prey to decrease the risk for breaking their relatively more delicate canine teeth (Gonyea, 1976; Therrien, 2005; Meachen-Samuels, 2012). The relatively robust jaws and long carnassial teeth of barbourofelids allowed them to generate significantly more powerful bites than felids of the small body size (Therrien, 2005).
Indeed, according to Meachen-Samuels (2012), Barbourofelis loveorum has one of the most exaggeratedly lengthened and flattened canines for a saber-toothed mammal and also has a high degree of associated postcranial adaptations associated with ambush predation. Their skeleton is highly robust, with numerous noticeable bony projections for muscle attachment throughout. Baskin (2005) calculated the average brachial index (or BI, which measures the ratio of the length of the radius to that of the humerus) of Barbourofelis loveorum to be 0.69, the average crural index (or CI, the ratio of tibia length to that of the femur) to be 0.74, and the average intermembral index (or IMI, the ratio of forelimb length to hindlimb length) to be 0.95. When compared to the BI, CI, and IMI of extant large felines and various extinct sabertooths as discussed in Gonyea (1976), Baskin (2005) noted that the values for Barbourofelis loveorum fall within the range assigned by the previous author as belonging to ambush predators. The species’ low BI and CI, along with its proposed subdigitigrade stance, may also be indicative that Barbourofelis loveorum preferred a thickly wooded habitat. In addition, the cross sectional area of the humerus of Barbourofelis loveorum is relatively large, with two specimens measured at 1028 mm2 and 1050 mm2, suggesting that Barbourofelis loveorum may have been able to bring down prey much larger than itself, perhaps even the likes of Teleoceras and Gomphotherium (Baskin, 2005). While the same morphological specializations for ambush predation may be speculated to have been adaptations for an arboreal lifestyle, this is unlikely for Barbourofelis loveorum because of their diminuitive tails and large body weight (Meachen-Samuels, 2012).

Having elongate sabers for upper canines presents an interesting problem developmentally for Barbourofelis loveorum, as large teeth tend to have comparatively large roots. Therefore, for many carnivores that possessed this character (like Smilodon and Homotherium), the permanent upper canines (C1) did not replace the smaller, more manageable deciduous upper canines (DC1) until the maxilla was large enough to support it. However, the DC1 of Barbourofelis loveroum (and other members of its genus) was about the size of the permanent canine tooth (Fig. 2). This implies, and is supported by fossil evidence, that the DC1 of Barbourofelis loveorum did not grow and erupt until relatively later in development; in fact, not until long after the permanent P4 began erupting (Fig. 4; Bryant, 1988). The lack of functional upper canine teeth in juveniles of Barbourofelis loveorum suggests several points of interest regarding its growth and development. Firstly, because they did not erupt until late in life, the large deciduous canines may have functionally served as a second set of canines that could be backed up by the real permanent canines should they have become broken when the individual was relatively young. Secondly, the kittens of Barbourofelis loveorum would have needed a relatively prolonged time of parental care (provided that their growth rate was similar to those of extant felines), as they would presumably not be able to learn to hunt for themselves until the DC1 fully erupted. Although speculative, this may have necessitated some form of “extended familial or social structure” to accommodate youngsters of Barbourofelis loveorum that could not hunt until well into development (Bryant, 1988). But this is somewhat contracted by its relatively small brain size, as highly social mammals tend to have larger brains.
Sources
- Original Author(s): Arianna Harrington
- Original Completion Date: September 14, 2012
- Editor(s) Name(s): Richard C. Hulbert Jr. and Natali Valdes
- Last Updated On: February 26, 2015
This material is based upon work supported by the National Science Foundation under Grant Number CSBR 1203222, Jonathan Bloch, Principal Investigator. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Copyright © Florida Museum of Natural History, University of Florida